Advertisement
Advertisement
CBSE Class 10 Science Solved Question Paper 2020

CBSE
•Science•2020PDFUnknown23 pages
CBSE Class X 2020 Science question paper with solution prepared by our expert teachers in PDF format. 10yearsquestionpaper.com has also complied previous year CBSE Science class 10 question papers here. Download the CBSE Science Question Paper 2020 with solutions will help students to score more marks in your CBSE final Examinations.
Page 1
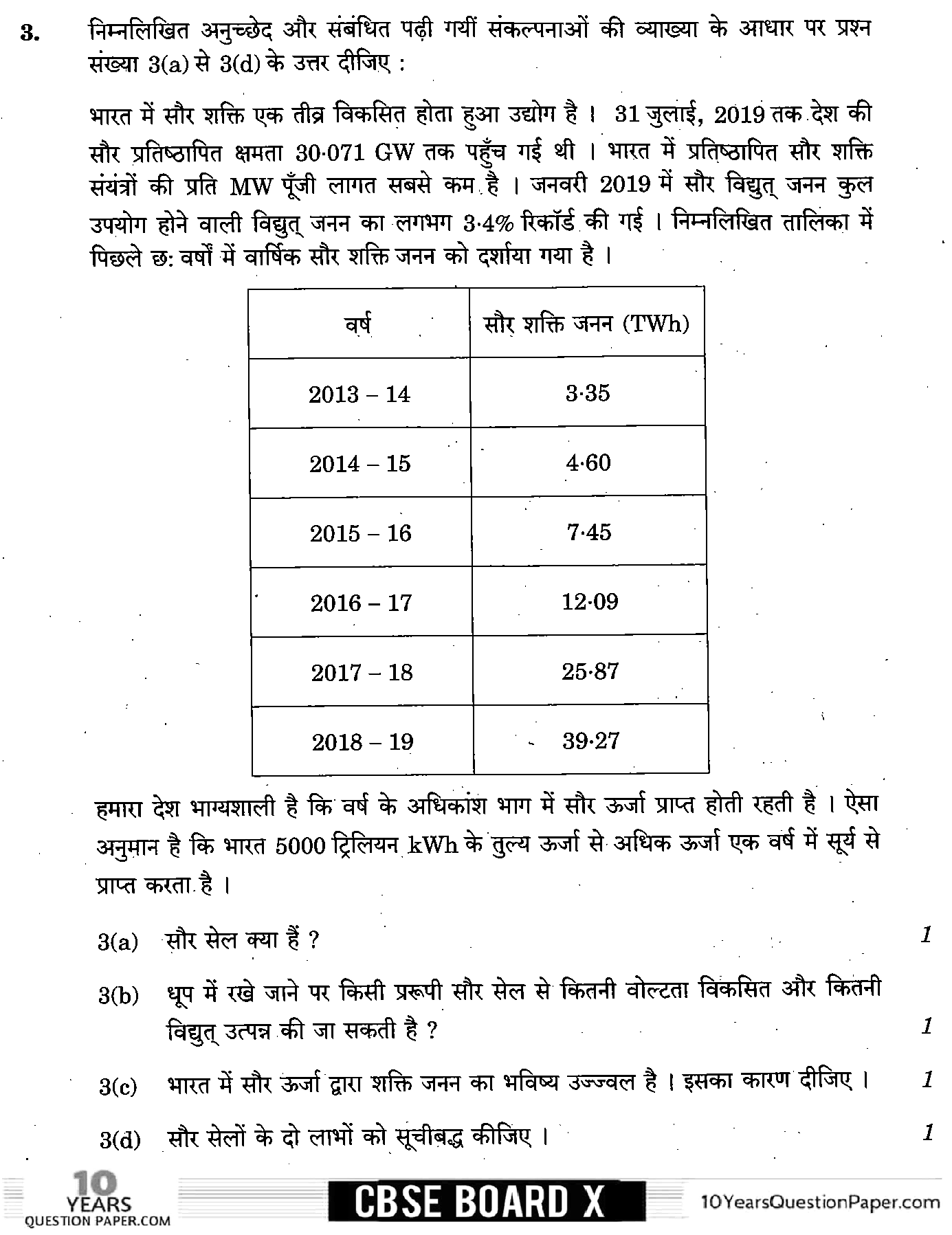
Page 2
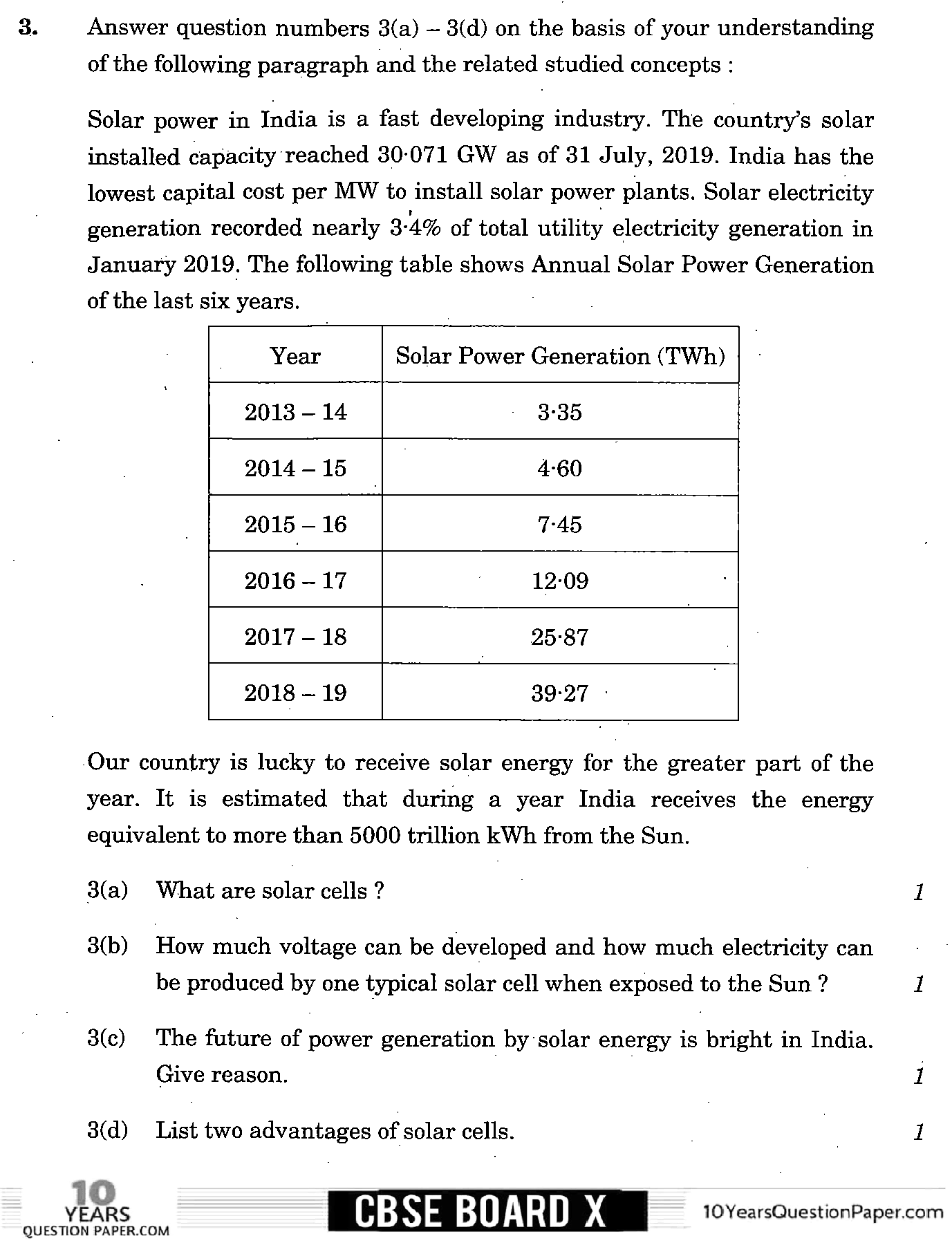
Page 3
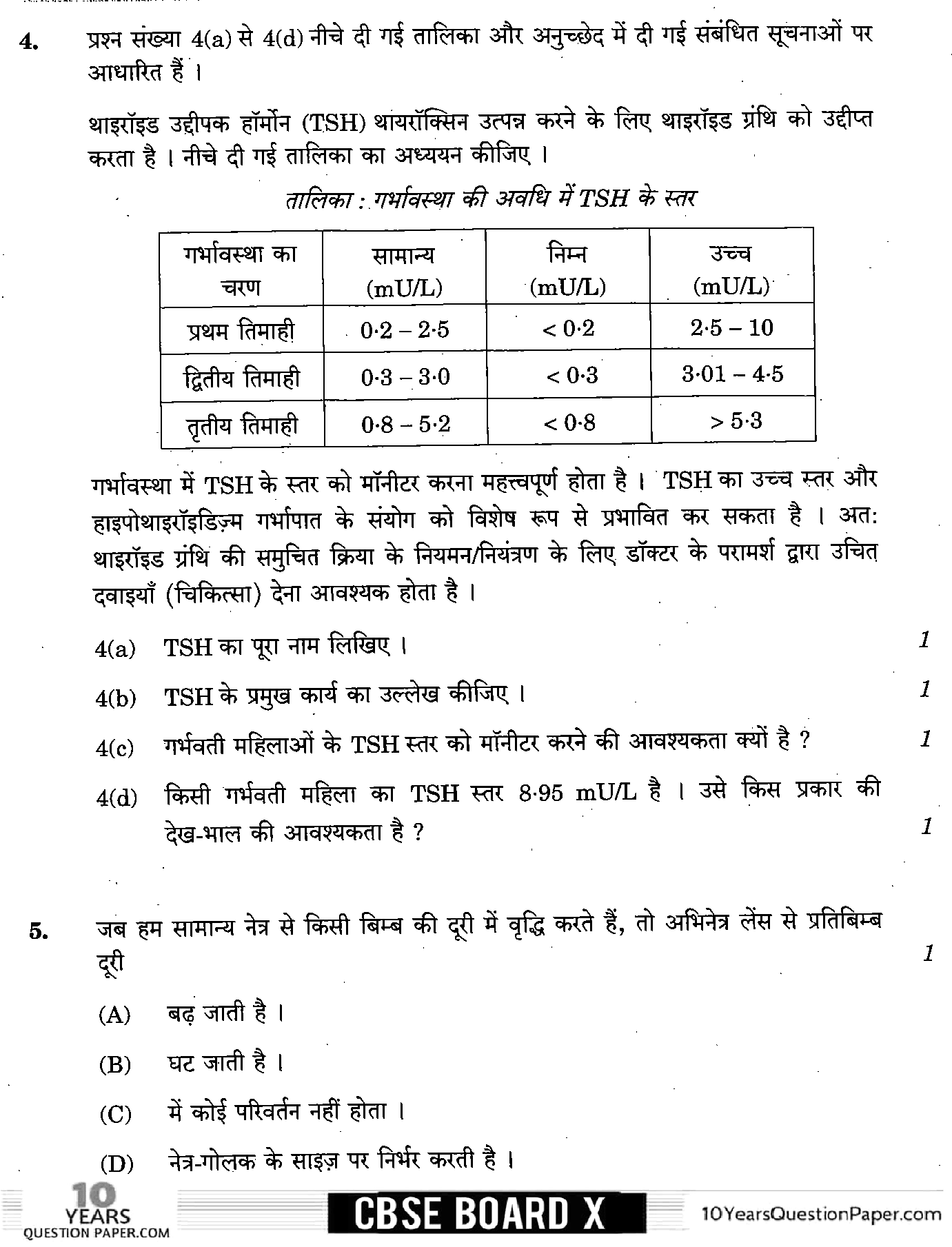
Page 4
Page 5
Page 6
Page 7
Page 8
Page 9
Page 10
Page 11
Page 12
Page 13
Page 14
Page 15
Page 16
Page 17
Page 18
Page 19
Page 20
Page 21
Page 22
Page 23
1 / 23
100%
Advertisement
Advertisement
Download
- Format
- Size
- Unknown
- Pages
- 23
Advertisement
Advertisement
Advertisement
Advertisement